The ATF4 protein acts in tumors and tumor-supporting fibroblasts to promote tumor survival
A study led by researchers at the university's Perelman School of Medicine suggests that a stress protein that is overactive in many types of cancer cells also plays a key role in tumor-supporting cells called fibroblasts, and it may be a good target for cancer therapies in the future. Pennsylvania.
The researchers, whose findings appear in Nature Cell Biology, have discovered in experiments with mouse models of pancreatic and melanoma cancer that the protein, known as ATF4, enables fibroblasts to support tumor growth by promoting the formation of tumor-serving angiogenesis.
The researchers found that deletion of ATF4 in fibroblasts severely impairs tumor-supporting new blood vessel formation as well as tumor growth, without causing significant harm to mice.
“Our results indicate that inhibiting ATF4 can work against many types of cancer, and we are now actively pursuing this strategy,” said senior study author Konstantinos Kominis, Ph.D., the Richard H. Chamberlain Professor of Oncology Research in the Division of Radiation Oncology. in Pennsylvania. "Every tumor we examined regulates ATF4."
The study's first author, who conducted most of the experiments, was Ioannis Virginia, MD, a senior research associate and adjunct assistant professor in the Communis Laboratory.
ATF4 is produced in cells as part of a broad response to stresses such as oxygen or nutrient deprivation. It acts as a master switch for the activities of hundreds of genes that help cells survive these stresses.
As the lab of Communis and others have shown in recent years, many types of tumors depend on the stress response associated with ATF4 to survive despite the intense stresses they cause themselves through their rapid growth.
The researchers began the new study by engineering mice whose ATF4 gene can be deleted throughout the body at any time. They found that if they delete ATF4 before or even after tumors begin to grow in mice, the growth of tumors and their ability to spread to distant organs is significantly impaired.
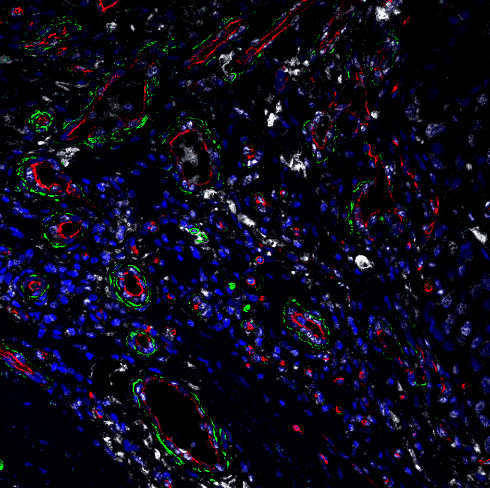
The scientists then used a powerful and relatively new technique called single-cell RNA sequencing to examine the effect of ATF4 deletion in all types of cells within a tumor — and observed a strikingly large effect on a group of tumor-supporting cells called cancer-associated fibroblasts (CAFs).
Fibroblasts are supportive cells found in nearly all organs, producing the primary structural protein collagen, promoting new blood vessel formation, and generally aiding in tissue repair and maintenance. Many types of tumors share proximal fibroblasts, transforming them into a CAF model in which the tumor is primarily supported.
However, the researchers noted that in ATF4-deficient mice, CAF often lacked the usual markers of activation, and was defective in collagen production and secretion of molecules that promote new vessel growth. As a result, the levels of collagen and tumor-feeding blood vessels were significantly reduced within the mice's tumors, causing the cancer cells to die.
When the scientists deleted ATF4 only in the fibroblasts, they saw a tumor-slowing affect roughly the same intensity that was observed with whole-body ATF4 deletion. And when the researchers added normal ATF4-containing fibroblasts to ATF4-deficient mice, the growth-slowing effect of ATF4 deletion was largely reversed.
"These findings indicate that ATF4 support for tumorigenesis is largely mediated by CAF," said Virginiadis.
Confirming the potential relevance of their research to human cancers, they found that in tumor tissues from human pancreatic cancer and melanoma patients, there was a significant association between markers of ATF4 activity and markers of collagen production. Moreover, in cases of melanoma, higher collagen production was associated with a worse outlook.
The researchers hope that targeting ATF4 will not have unacceptable side effects, because mice in which the gene was deleted in adulthood showed only modest, temporary weight loss and other minor abnormalities.
"Overall, ATF4 appears to be an attractive target for cancer," Communis said. “A drug that inhibits it will block its pro-tumor effect not only in cancer cells but also in cancer-associated fibroblasts, so it should be a double whammy for the tumor. But we are still a few years away from that.”
Koumenis' lab is now developing ATF4 inhibitors that could be tested in further animal studies and eventually in human cancer patients.
The research was supported by the National Institutes of Health (P01 CA165997, 1R01CA268597, P01 CA217805, UL1TR001878)
Source:
Materials provided by University of Pennsylvania School of Medicine. Note: Content may be edited for style and length.
Reference:
Ioannis I. Verginadis, Harris Avgousti, James Monslow, Giorgos Skoufos, Frank Chinga, Kyle Kim, Nektaria Maria Leli, Ilias V. Karagounis, Brett I. Bell, Anastasia Velalopoulou, Carlo Salas Salinas, Victoria S. Wu, Yang Li, Jiangbin Ye, David A. Scott, Andrei L. Osterman, Arjun Sengupta, Aalim Weljie, Menggui Huang, Duo Zhang, Yi Fan, Enrico Radaelli, John W. Tobias, Florian Rambow, Panagiotis Karras, Jean-Christophe Marine, Xiaowei Xu, Artemis G. Hatzigeorgiou, Sandra Ryeom, J. Alan Diehl, Serge Y. Fuchs, Ellen Puré, Constantinos Koumenis. A stromal Integrated Stress Response activates perivascular cancer-associated fibroblasts to drive angiogenesis and tumor progression. Nature Cell Biology, 2022; DOI: 10.1038/s41556-022-00918-8
.png)